Science: Coin Battery
Hello everyone. This is Bill from the Okanagan Regional Library System. Welcome to the fun and inventive world of making STEAM projects in your own home. With this STEAM project, you will be able to build your own Coin Battery.
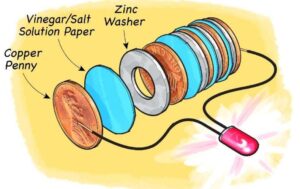
We use batteries to power many small devices – but how do they product electric current? There is no better way to understand how batteries work then to build your own. In this project, you will build a battery made of coins, washers, and pieces of dishcloth – and use it to power a set of fairy lights.
Materials Needed:
- Pencil
- Scissors
- Spoon
- Small bowl
- Table Salt
- Vinegar
- Paper Towels
- 10 Galvanize washers
- 10 Pennies
- 1 Crocodile Clip Wire
- Rubber band
- Set of Fairy lights or LED Circuit Lights
- Absorbent Cloth
Time: 30 minutes
Steps:
1. Pour about 30 ml of vinegar into the small bowl. Keep adding salt and stirring until no more salt will dissolve.

2. Place the pennies into the salt and vinegar solution. Leave them for about 5 minutes, until they become shiny. Remove and dry them with a paper towel, then wash and dry your hands.


3. Using a coin as a template, cut out 10 circles from the absorbent cloth.

4. Place the disks of absorbent cloth into the dish of salt and vinegar solution, and let them soak a few minutes. The vinegar will be the electrolyte in your battery.

5. Lay down a galvanized washer, stack a cloth disk on top, and then a copper coin on top of that. Repeat in this order until all the materials are in a neat pile, making sure that the top disk is a coin. Wrap the entire column with the rubber band. Note: The tighter the battery pack is held together, the stronger the electric current will be.


6. Attach one crocodile clip wire to each end of your battery, using the rubber band to hold everything in place. Make sure that the stripped wire ends are tucked firmly under the rubber band touching the metal on each end.

7. Clip each of the crocodile clips to one of the exposed copper posts in the fairy light battery case. The fairy lights should now illuminate.

The Science behind your Coin Battery
A battery converts chemical energy into electrical energy through a chemical reaction. In each cell, the zinc washer (called the anode) produces electrons, as zinc atoms dissolve in the salt and vinegar solution (the electrolyte). Electrons flow through the wires, and are taken in at the copper end (the cathode), were they take part in another chemical reaction in the electrolyte. The voltages of all the cells add up, giving the electrons that leave the battery at the bottom enough energy to light the LEDs.
Real World Science – The First Battery

Italian scientist Alessandro Volta invented the battery in 1799. Just like the battery you have built, it was made with copper and zinc disks. Volta used cloth disks soaked in brine (salty water), rather than disks of dishcloth soaked in salt and vinegar.
STEAM
This activity includes everything you need for a comprehensive STEAM project.
Science: Understanding how electric currents in a battery are created.
Technology: Understanding how batteries are used in real life situations.
Engineering and Art: Construction of the Coin Battery.
Math: Measuring and cutting out the parts needed to construct your coin battery.




